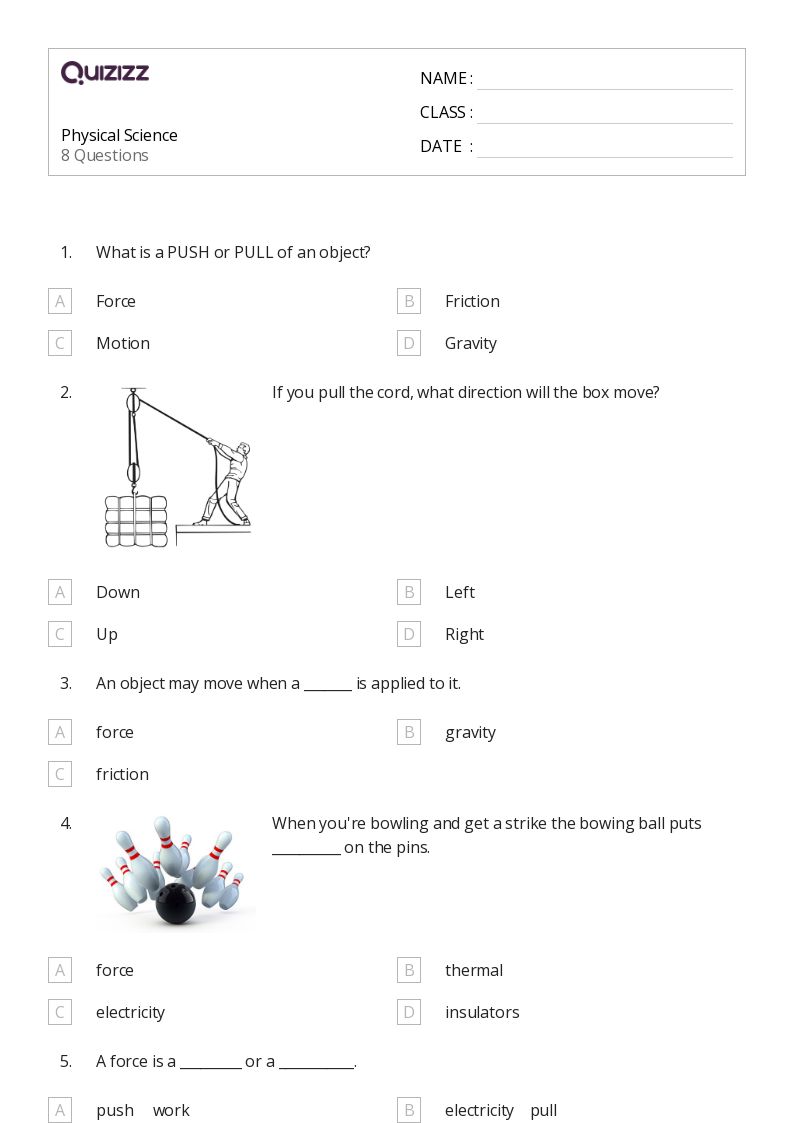
The definition of products in physical science is a crucial concept that underpins various scientific disciplines, including chemistry, physics, and environmental science. Understanding what products are and how they interact within different systems is essential for students, educators, and professionals alike. Products are the substances that result from chemical reactions or physical processes, and they play a significant role in the study of matter and energy transformations.
In this article, we will delve deep into the definition of products in physical science, exploring their significance, examples, and how they differ across various scientific fields. We will also discuss the properties of products and their relevance in real-world applications, including industry, everyday life, and environmental science.
By the end of this article, you will have a comprehensive understanding of products in physical science, along with their implications and applications. Whether you are a student preparing for exams or a professional seeking to refresh your knowledge, this guide aims to provide valuable insights into this fundamental concept.
Table of Contents
- What are Products?
- Importance of Products in Physical Science
- Examples of Products
- Products in Chemical Reactions
- Products in Physical Reactions
- Properties of Products
- Real-World Applications of Products
- Conclusion
What are Products?
In physical science, products refer to the substances that are formed as a result of a chemical reaction or physical process. When reactants undergo a transformation, they yield products, which can be either new substances or changes in the state of the original materials.
For example, when hydrogen gas reacts with oxygen gas, water is formed as a product. This transformation is represented by the chemical equation:
2H2 + O2 → 2H2O
In this equation, hydrogen and oxygen are the reactants, while water is the product. Understanding this distinction is vital for grasping the principles of physical science.
Importance of Products in Physical Science
Products play a significant role in various scientific disciplines for several reasons:
- Understanding Reactions: Products provide insight into how reactants interact and the nature of chemical bonds.
- Predicting Outcomes: By knowing the products, scientists can predict the behavior of substances in different conditions.
- Applications in Industry: Many industrial processes rely on the formation of specific products for the production of goods.
- Environmental Impact: Understanding products helps assess the environmental consequences of chemical reactions.
Examples of Products
Products can be observed in various scientific contexts. Here are some examples:
1. Chemical Products
In chemistry, products are formed during chemical reactions. Common examples include:
- Combustion of hydrocarbons producing carbon dioxide and water.
- Neutralization reactions yielding salt and water.
- Photosynthesis, where plants convert carbon dioxide and water into glucose and oxygen.
2. Physical Products
In physical sciences, products can also refer to changes in states of matter. For instance:
- Ice melting into water.
- Water vapor condensing into liquid water.
Products in Chemical Reactions
Chemical reactions are fundamental processes in physical science. They involve the transformation of reactants into products through the breaking and forming of chemical bonds. Understanding the nature of products formed in these reactions is crucial for various applications.
Key points to consider include:
- Balancing equations to determine the quantity of products formed.
- The role of catalysts in altering reaction pathways and product yield.
- Energy changes associated with the formation of products (exothermic vs. endothermic reactions).
Products in Physical Reactions
Physical reactions differ from chemical reactions as they do not involve changes in chemical composition. However, products still emerge from these processes. Examples include:
- Phase changes, such as melting, freezing, and sublimation.
- Mixing substances to form homogeneous or heterogeneous mixtures.
Understanding the products of physical reactions is essential in fields such as materials science and engineering.
Properties of Products
The properties of products can vary significantly based on their composition and the conditions under which they are formed. Key properties to consider include:
- Physical State: Products can exist in solid, liquid, or gas states depending on temperature and pressure.
- Reactivity: Some products may be more reactive than their reactants, affecting their stability.
- Purity: The presence of impurities can alter the properties of products, influencing their efficacy in applications.
Real-World Applications of Products
Understanding products in physical science has practical implications across various fields:
- Pharmaceuticals: Knowledge of chemical products is essential for drug development.
- Environmental Science: Assessing the products of chemical reactions helps evaluate environmental impacts.
- Manufacturing: Industrial processes rely on the formation of specific products for the production of materials.
Conclusion
In conclusion, the definition of products in physical science encompasses a wide range of concepts and applications. Understanding products is vital for grasping the principles of chemical and physical reactions, their properties, and their implications in real-world scenarios. We encourage readers to explore this topic further and engage with the scientific community by sharing their thoughts in the comments below or reading related articles on our site.
Thank you for reading! We hope you found this article insightful and informative. Stay curious and continue your journey of learning in the fascinating world of physical science.